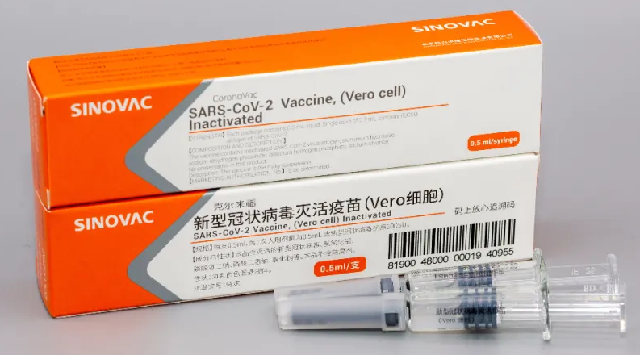
Phase I tested safety, with none of the participants reporting any severe side effects from the vaccine.The company to begin Phase III trials the last step before it is approved for public use
Sinovac Biotech Ltd. based in Beijing, China, said its vaccine called CoronaVac found safe and positive early data from human trials, Sinovac said in a press release on Sunday.
More than 90% of people who received the vaccine have induced neutralizing antibodies two weeks after inoculation. The shot hasn’t caused severe side effects.
The trial was randomized, double-blind, and placebo-controlled. 743 healthy people between ages 18 to 59 have participated in the trial conducted at the Jiangsu Provincial Center for Disease Control and Prevention, Eastern China.
Out of 743 participants, 143 are included in Phase I, testing the safety of the immunization, which is a dead strain of the virus.
All others are in Phase II, seeing if antibodies would be triggered after being given two shots in 14 days.
The company says another group in the study will be given the shots on a 28-day interval to see if that is more effective.
The company also announced earlier this month a partnership with company Instituto Butantan to conduct its phase III trial in Brazil. The South American country has become the second-largest outbreak of COVID-19 in a global pandemic that so far infected more than 7.7 million people and killed over 428,000.
Read more about: China, Coronavirus, Coronavirus Vaccine, COVID-19, Pandemics