FDA approval is the first to be received for the treatment of addictions among all TMS systems and represents the third FDA approval that Brainsway receives
The dual company Brainsway reports a U.S. Food and Drug Administration (FDA) approval of its non-invasive treatment of brain disorders, to be used as a short-term smoking cessation tool for adults with smoking addiction.
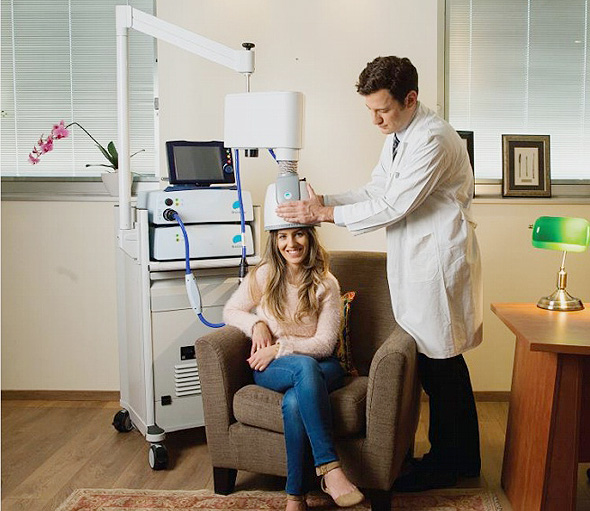
The treatment includes sitting in a chair and wearing a helmet comprising a patented H-coil for 20-minutes. The coil stimulates the brain by creating a temporary magnetic field that creates stimulation or inhibition of neurons deep inside the brain.
Nicotine addiction, similar to drug and alcohol addiction, involves changes in the reward system in the brain, and an uncontrollable need to smoke. About 38 million adults in the United States smoke cigarettes and about 480,000 die as a result of smoking each year.
Smoking is one of the leading causes of death in the United States, and a leading cause of other serious health events such as lung cancer and heart disease. “Although other medical measures exist, a substantial medical need still exists for treatment that can prolong the duration of withdrawal among smokers,” said Dr. Christopher von Jako, President, and CEO of Brainsway.
Based on the results from a pivotal, large, randomized trial Brainsway conducted on 262 patients, the company is confident that Deep TMS technology will play an important role in treating smokers seeking to quit.
“We look forward to the commercial and controlled launch in the United States of our Deep TMS system that includes an H4 coil for this medical indication early next year,” von Jako said. “The recent approval from Brainsway positions it as a market leader, and reiterates our commitment to leverage the technology platform for innovative and advanced treatment solutions for patients with a wide range of diseases,” he added.
The efficacy of the Brainsway Deep TMS system (H4 coil) for use as a short-term smoking cessation method was demonstrated in an experiment that included 262 patients randomly divided into two groups: an active treatment group treated with the Brainsway H4 coil, aimed at stimulating addiction-related neural networks, and a control group received a sham treatment (placebo).
The treatments were performed daily, 5 days a week, for 3 weeks, as well as an additional 3 weeks of follow-up with treatment once a week (so that 18 treatments were performed over 6 weeks). The main objective of the experiment was to compare the two groups at a 4-week continuous smoking cessation rate. The experiment showed a positive treatment result and גemonstrate significant efficacy in the effect on smoking cessation.
In addition, the average number of cigarettes a patient smoked per day, from the beginning of the experiment until the end, decreased statistically significantly, in the group treated with Deep TMS compared to the control group.
According to von Jako, this is the first FDA 510(k) clearance in the addictions market among all TMS devices, and it represents BrainsWay’s third FDA-approval for the coil and indication, following the clearance of the H1-coil for patients suffering from a major depressive disorder and the H7-coil as an adjunct therapy for the treatment of patients with obsessive-compulsive disorder (OCD).
Read more about: brain disorders, Brainsway, Deep TMS system