Imagine getting treatment for a perfectly healthy young heart that would allow it to recover from an otherwise devastating injury decades later.
This seems very farfetched; after all, cardiovascular diseases that are humanity’s No. 1 or No. 2 cause of death (after cancer), aren’t generally regarded as something one can prepare for with preventive treatment.
Until recently, Prof. Eldad Tzahor, whose lab at the Weizmann Institute of Science in Rehovot studies heart-tissue regeneration, had also considered it science fiction.
But he and his lab colleagues have now activated a cellular mechanism in healthy mouse hearts that makes these mice resilient to future heart attacks – even when they occur months later. The preventive procedure performed on healthy mice improved their recovery from later-occurring cardiac injury.
When will this procedure be available to humans?
The procedure is many years from being applicable to humans, the researchers stressed. But their findings, just published in the prestigious Nature Cardiovascular Research, reshape our understanding of the regenerative capabilities of the heart and possibly other organs and how they might be enhanced through preventive medical intervention.
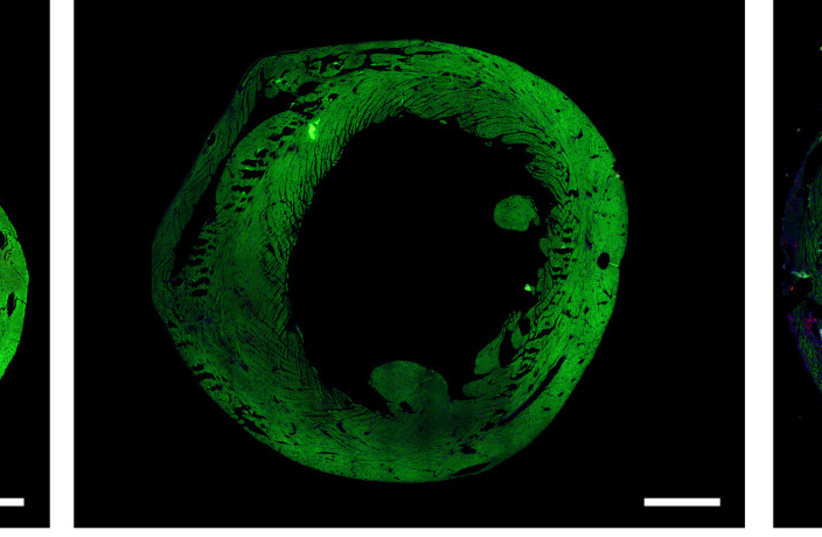
The heart of a mouse whose ERBB2 gene had been temporarily activated (center) contracts properly after injury, similarly to an uninjured heart (left)
“It’s a proof of concept,” said Tzahor, “and it points to new avenues of research that examine giving heart treatment not only after the damage happens, but from a preventive position that increases the capacity for recovery from an injury before the damage even occurs.”
The study, led by Dr. Avraham Shakked in Tzahor’s lab in Weizmann’s molecular cell biology department, focused on genetically engineered mice whose cardiomyocytes – the cells that make up the heart muscle tissue – overexpress a gene that triggers cell division in mice and other mammals, including humans. In previous studies scientists in Tzahor’s lab had found that the gene, ERBB2, causes cell division in cardiomyocytes – a remarkable occurrence because at around the time of birth these cells lose their capacity to multiply.
“During fetal development, our cells are assigned their different roles – nerves, cornea, heart muscle and the like – through a process called differentiation,” Shakked noted. “It’s characterized by a spectrum – on one end are stem cells, which are undifferentiated but capable of dividing and producing various types of cells, while on the other end are highly-specialized cells like cardiomyocytes that are no longer able to divide after becoming differentiated. They’re very effective in their function, but the tissue they comprise does not regenerate naturally.”
This is one of the reasons that cardiac episodes are so devastating. Heart attacks kill off massive numbers of cardiomyocytes that the body cannot regenerate, so even those who survive the attack are often left with reduced cardiac performance.
When in previous studies, Tzahor’s team had managed to trigger the division of cardiomyocytes by switching on ERBB2 briefly in these cells; overall heart function actually decreased temporarily instead of improving straightaway. This happened because the ERBB2-expressing cardiomyocytes underwent dedifferentiation, meaning that they reverted to a less specialized state, closer to that of a fetal heart. This, in turn, limited their ability to contract, which is needed for proper heart function. But once the overexpression had stopped, the cardiomyocytes underwent redifferentiation – that is, they became highly specialized again – and cardiac performance improved.0
“The data made our jaws drop. We had found a cardiac fountain of youth in those mice, a novel way of making the heart younger and stronger.”
Professor Eldad Tzahor, Weizmann Institute of Technology
In the study, the scientists sought to understand what happens to hearts “rejuvenated” by ERBB2 and how, exactly, they redifferentiated and returned to normal function once the gene had been switched off. The performance of such hearts was indistinguishable from that of the control group, but Shakked did notice some significant differences in gene expression between the two populations.
“It was surprising and curious,” he recalled. “We had assumed that everything returns to normal after ERBB2 is switched off in the cardiomyocytes. Yet here we were, seeing a different genetic pattern – overexpression in some genes and underexpression in others – following ERBB2 activation. In other words, we found long-term effects.”
This discovery made Shakked and Tzahor wonder whether ERBB2 expression could be calibrated for improved cardiac performance. “It made us think that ERBB2 wasn’t just a switch that prevents differentiation, but part of a mechanism that could make the heart younger and more resilient,” Tzahor added/
To test this hypothesis, the researchers reversed the order of their previous experiments with ERBB2. Instead of switching ERBB2 on in injured mice to make their cardiomyocytes divide, they first switched it on in healthy mice for a few weeks and then switched it off again. Next, the researchers observed how the hearts of those mice coped with an injury. The result was that mice that had been made to overexpress ERBB2 recovered but others didn’t. “The data made our jaws drop,” Tzahor said. “We had found a cardiac fountain of youth in those mice, a novel way of making the heart younger and stronger.”
The researchers are now examining a number of hypotheses about mechanisms through which a brief overexpression of ERBB2 could help mice survive future heart damage. One possibility is that the gene triggers a series of changes that allow more cardiomyocytes to survive the lack of oxygen which is characteristic of heart attacks and is particularly destructive for cardiomyocytes.
They also revealed how a negative feedback loop drives redifferentiation in cardiomyocytes. “The body makes sure that differentiation happens because, generally, it doesn’t want cells that do nothing but divide, like cancer cells,” Tzahor said. “That’s why ERBB2, while moving cardiomyocytes in the opposite direction of natural differentiation, simultaneously activates genes that trigger differentiation. There are checks and balances in place. If it weren’t for this mechanism, the dedifferentiated heart cells would not have been able to redifferentiate back to functioning cardiomyocytes.”
The team found that a mouse whose ERBB2 had been temporarily activated when it was three months old recovered from a major cardiac injury that happened five months later. “If we translate this to human years, it’s comparable to an 18-year-old getting treatment that allows that person to survive a heart attack at age 50!” Tzahor exclaimed.
Yet this kind of treatment is at present nowhere near being applicable to human beings. “We’re lowering the function of cardiomyocytes to allow them to be restored in the future,” Tzahor explains. “From a clinical perspective, this is an extreme and drastic intervention. Still, at least in principle, our research might lead to a way of treating people who have a high risk of heart attack, before these attacks even happen.”