The watch monitors detect fever, respiratory and chronic diseases as well as heart arrhythmias at a uniquely precise level of an ECG device
Israeli company CardiacSense, the developer of a medical watch for remote patient vital signs monitoring and detection of fever, heart arrhythmias, and chronic diseases, has signed an exclusive distribution agreement in India worth at least $32.4 million. This brings the company’s total backlog of orders to more than $60 million.
As part of the agreement, CardiacSense will supply 150,000 medical watches for use in hospitals, home hospitalizations and monitoring chronically ill patients over the next 4 years, to Xplore Lifestyle Solutions, which has been operating in India for the past 40 years as a manufacturer and distributor of medical rehabilitation products.
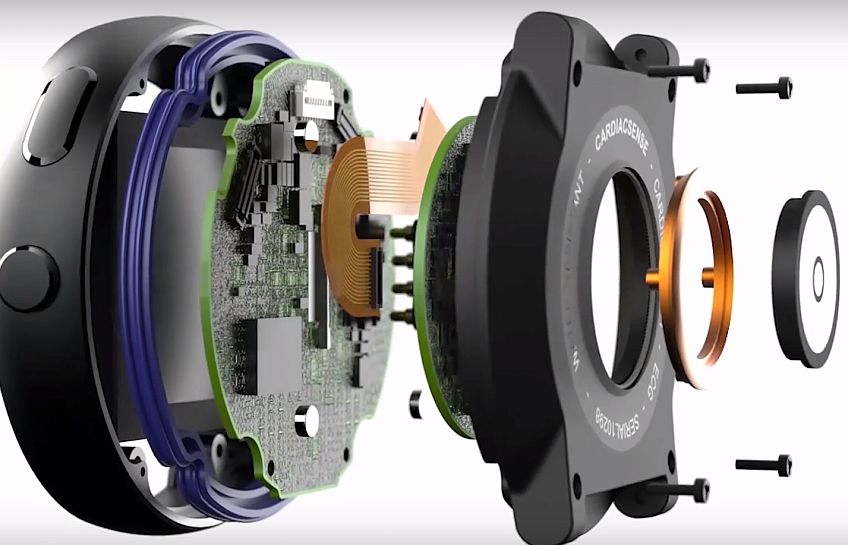
CardiacSense’s watch uses a combination of patent-protected technology of optical indicators with temperature, movement, and pressure sensors in order to monitor the vital physical measurements of patients and transmit data directly to their personal physicians. Among other things, the watch measures respiratory rate, heart rate, blood pressure and blood oxygen saturation and detects cardiac arrhythmias and heart failure at the uniquely precise level equivalent to an ECG.
CardiacSense is responding to the demand for remote monitoring of diseases and the status of diagnosed diseases of patients in clinics and hospitals. This need is characterized by the growing global trend to switch to online community digital healthcare, which this year has become necessary due to the coronavirus crisis.
CardiacSense founded in 2009 by CEO Eldad Shemesh.
Shemesh said, “The agreement in India is a major business success, demonstrating the international recognition that our solution, for constant monitoring, is the optimal technology for a range of diagnoses. Supporting this is the fact that our clinical results speak for themselves with unprecedented success in precise measuring, to a level of 99%, of heart arrhythmias using a wearable medical wristwatch, as part of a clinical trial conducted in three medical centers in Israel and abroad.”
He added, “At the same time as signing distribution agreements in Argentina, Spain, Turkey, Uruguay, Australia, South Africa, and Chile, in the past months, we have submitted all that’s required to receive FDA and CE approvals in order to also enter into major agreements in the US and Europe. We believe that we are only at the beginning of the road, and we are working to commercialize our solution in additional territories and markets.”
Read more about: CardiacSense