The Health Ministry has approved the expanded use of an innovative COVID-19 treatment that helped 15 out of 17 severe patients who took it to be released from the hospital one day after receiving their final dose.
The drug, MesenCure, has been tested by Rambam Medical Center as part of a Phase I/II trial. The ministry has approved allowing any interested Israeli hospital to take part in the Phase II trial and to use the drug for additional approved patients.
The goal of the expanded trial, which will include a minimum of 50 patients, is to confirm the safety and efficacy of the drug, which was developed by Bonus BioGroup.
MesenCure, which consists of activated Mesenchymal Stromal Cells (MSCs) that are isolated from the adipose tissue of healthy donors, was found to reduce inflammation and alleviate respiratory and other symptoms in patients suffering from life-threatening respiratory distress brought on by COVID-19.
if(window.location.pathname.indexOf(“656089”) != -1){document.getElementsByClassName(“divConnatix”)[0].style.display =”none”;}else if(window.location.pathname.indexOf(“/israel-news/”) != -1){ document.getElementsByClassName(“divConnatix”)[0].style.display =”none”; var script = document.createElement(‘script’); script.src = ‘https://player.anyclip.com/anyclip-widget/lre-widget/prod/v1/src/lre.js’; script.setAttribute(‘pubname’,’jpostcom’); script.setAttribute(‘widgetname’,’0011r00001lcD1i_12258′); document.getElementsByClassName(‘divAnyClip’)[0].appendChild(script);}
Back in May, the company reported on 10 COVID patients between the ages of 45 to 75, all with severe symptoms. Ninety percent of them also had comorbidities.
The data showed a 40% decrease in lung inflammation from treatment – from 55% to 15%, as seen in chest X-rays, in the first five days after treatment. One month later, lung inflammation reached 1%.
Additionally, patients showed significantly improved respiratory function, with blood oxygen saturation increasing to 95% and lung functioning returning to almost entirely normal levels after only one month.
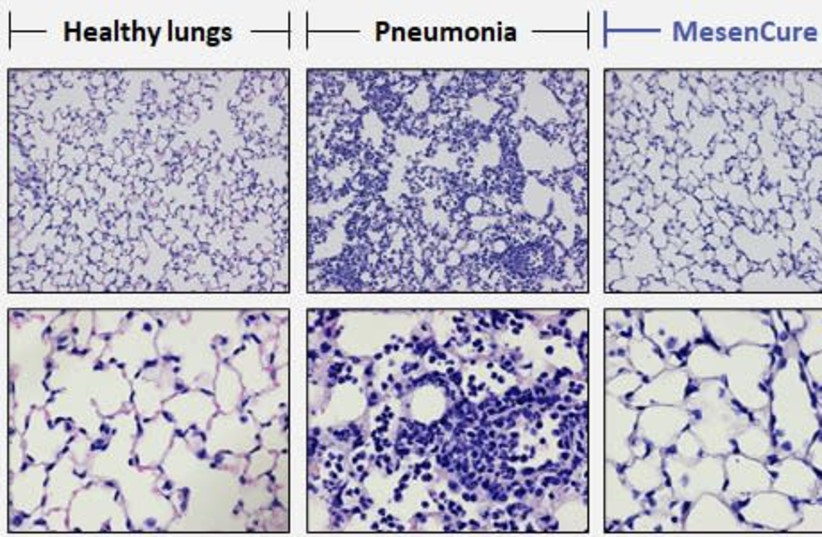
The company’s CEO Shai Meretzki had shared a laboratory image of a healthy lung, a sick lung and lung treated with MesenCure with The Jerusalem Post in May.
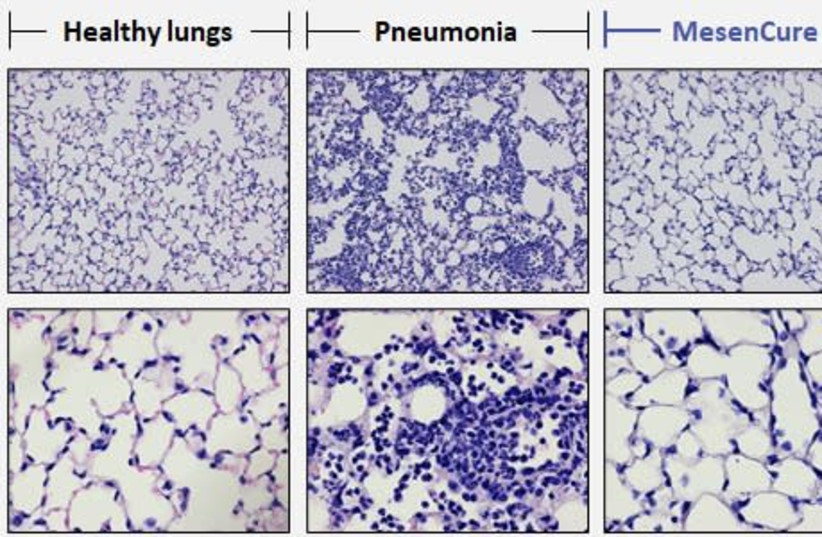 A laboratory image shows a healthy lung, a sick lung and lung treated with MesenCure. (credit: Courtesy)
A laboratory image shows a healthy lung, a sick lung and lung treated with MesenCure. (credit: Courtesy)“The treated lung looks almost identical to the normal, healthy lung – complete healing, complete prevention of damage to the lung,” Meretzki said.
Most strikingly, patients were discharged from the hospital after a median duration of only one day following the treatment.
Since then, six additional patients received MesenCure, bringing the numbers to 15 out of 17 who were released – 88%.
Bonus was founded in 2008. It has been working with MSCs for a decade from its Haifa headquarters, where it developed its core product, a tissue-engineered bone graft that is also based on MSCs.
When the coronavirus outbreak started in early 2020, Bonus started investigating the potential of MSCs to possibly reduce the cytokine storm in COVID-19 patients.
The company said that the drug could also be used to treat other, similar indications, including, lower respiratory tract infections, asthma and chronic obstructive pulmonary disease, which together, represent a global market expected to exceed $43 billion per year by 2026.