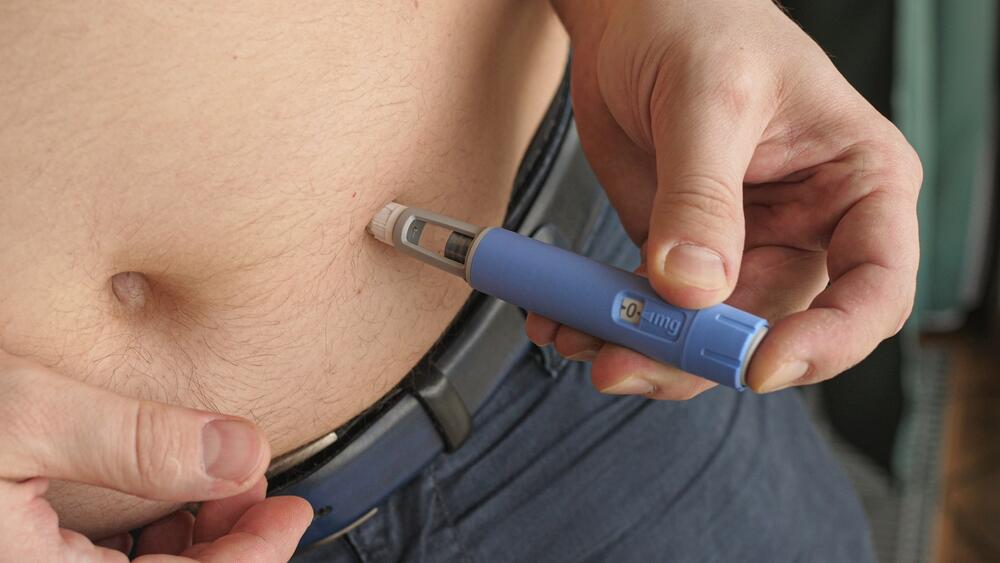
Following Wegovy’s arrival in Israel, the Health Ministry has today instructed pharmacists nationwide to cease selling Ozempic for weight loss, limiting its use to diabetes patients only. Those who have begun using Ozempic for weight loss are advised to gradually transition to Wegovy.
The frenzy over Ozempic in Israel began a little over two years ago, following a study in the US on Wegovy, which resulted in significant weight loss—more than 20% of body weight. The weekly Wegovy dose that led to this weight loss is 2.4 milligrams. Initially, only Ozempic was approved in Israel, available in a 1 milligram dose and intended solely for diabetic patients to manage blood glucose levels. However, it also led to a sense of fullness and a weight loss of approximately 14%, according to patient reports.
Since then, there has been a rush for Ozempic: Israelis with obesity received special authorization (form 29C) to use Ozempic, though they were not diabetic, leading to a nearly year-long depletion of Ozempic stocks and a severe shortage for the diabetic patients it was meant for. As reported by Maariv, the Health Ministry approved Wegovy’s marketing in Israel last June, and the drug arrived in Israel about two weeks ago, available by special order in most pharmacies.
Upon prescription, the drug typically arrives in the necessary dosage within a few days. Consequently, the Health Ministry has decided against further exceptional use of Ozempic for weight loss on Wednesday and has instructed pharmacists not to sell the drug any longer. Those with an existing authorization for Ozempic can continue to receive it until their authorization expires, usually after 6 months. New patients with obesity will now need to obtain a prescription for Wegovy injections.
Those already on Ozempic can finish course of treatment
According to the new directive issued by Eli Marom, Deputy Director of the Pharmacy Division at Israel’s Health Ministry said: “Section 47A of the Pharmacy Ordinance stipulates that no person shall produce or market a preparation, or prescribe its use, unless it is a registered preparation and in accordance with the registration and this Ordinance.
“With a therapeutic alternative now registered and marketed for the desired indication, it is not permissible to continue prescribing Ozempic for the unregistered indication of obesity treatment. Patients undergoing Ozempic treatment for obesity should consult their treating physician to switch to a registered medication. Patients with an active form 29C online can continue their treatment until the end of the approval period, to avoid disrupting the treatment continuity.”
Under the new guidelines, those who have not yet received weight loss injections will start Wegovy at an initial dose of 0.25 milligrams, gradually increasing in dose once a month or two until reaching the final dose of 2.4 milligrams once a week. According to medical literature, discontinuation of treatment typically leads to regaining weight, suggesting that the injection might need to be taken for life. The cost of the injection at the initial dose is NIS 600 per month, and at the final dose of 2.4 milligrams, it is NIS 1,200 per month.