Limaca Medical Is Working On Genetic Based Early Detection Disease testing
Limaca Medical has a new device called Precision.
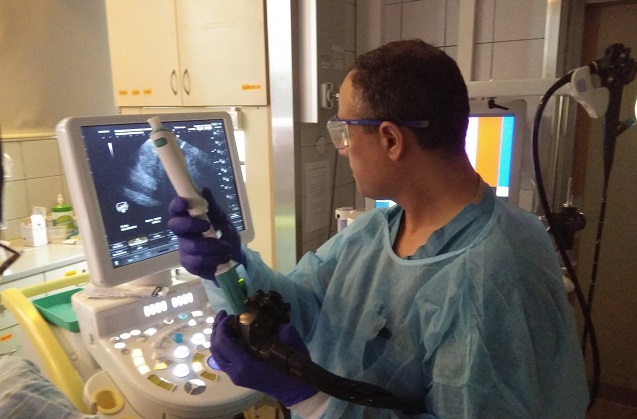
Israeli firm Limaca Medical offers an endoscopic ultrasound-guided biopsy for precision medicine. Aptly and simply named “Precision,” the company describes its new device as the next generation endoscopic ultrasound-guided biopsy device for precision medicine.
The company states that its device is used for improved diagnosis of the gastrointestinal system and adjacent organ tumors. It uses an automated, electro-mechanical revolving needle, provides high quality core tissue samples, improved diagnostic accuracy, and enables greater patient-specific treatments.
A portfolio company of The Trendlines Group, Lima has closed $1.25 million of a $1.5 million round to complete first-in-human (“FIH”) procedures, post-market clinical studies, and obtain regulatory approvals.
You have probably heard about the recent death of beloved Jeopardy host Alex Trebek. Trebek revealed a few years ago that he was suffering from pancreatic cancer.
Pancreatic cancer is the hardest to treat and a diagnosis is usually a death sentence. The reason for this is that cancer of the pancreas is so hard to detect that by the time it is uncovered the cancer has usually spread. So the cancer is detected elsewhere in the body only after the damage to the pancreas has been done.
Pancreatic cancer has a survival rate of just 9%. Limaca’s Precision utilizes genetic profiling through the use of more advanced biopsies.
[embedded content]
So by checking the genetics doctors can see if someone is at risk for a specific cancer and learn earlier what course of treatment to follow.
Limaca’s CEO Assaf Klein commented, “We designed Precision to improve upon endoscopic biopsies and make a substantial leap forward in this critical diagnostic procedure to advance precision treatment and achieve superior outcomes for cancer patients.”
Carl Rickenbaugh, Limaca’s Chairman, said, “The medical provider and payor markets respond to superior devices when they demonstrate strong clinical relevancy. We built Limaca to address the need for a more efficient endoscopic procedure for the provider, and for the cancer patient and payor, greater certainty to gain sufficient sample quality and quantity needed for pathology as well as more stringent genetic testing requirements.”



