Scientists at Tel Aviv University observed changes in healthy lung tissue that indicate the tissue is preparing for supporting the growth of disseminated breast cancer cells.
The changes were discovered in the tumor’s “microenvironment,” notably in connective tissue known as fibroblasts. According to the researchers, these changes in the tissues are a precursor to the development of spread malignant cells – metastases. According to them, a better knowledge of the metastatic process and its early detection may result in life-saving preventative treatment.
The researchers reported their findings in the journal eLife.
The researchers note that patients do not always die from the main tumor in many forms of cancer, including breast cancer. The primary cause of death is metastases, which invade and multiply in vital organs. Metastases may develop several years after patients have received all available therapies, including surgical removal of the initial tumor, followed by chemotherapy and radiation to eliminate any residual malignancy. Today’s follow-up methods detect metastases only when they are quite large — when the disease has progressed beyond the point where medicine can offer a cure.
Prof. Neta Erez, Chair of the Department of Pathology at the Sackler Faculty of Medicine, and her colleagues are examining the ‘black box’ – the time interval between apparent recovery and the appearance of metastases – in order to better understand the metastatic process and to detect it early. Their recent research has showed that designated tissues in organs where metastases are expected to arrive ‘prepare the space’ for their arrival and create a friendly environment for them long before the metastases themselves appear.
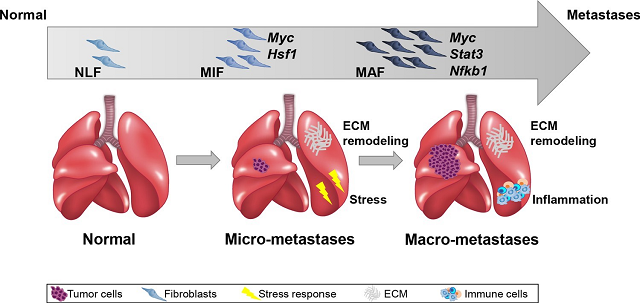
The current study, coordinated by Prof. Erez, looked for indicators of these changes that could be used to pinpoint the start of the process in the future. The researchers concentrated on connective tissue cells called fibroblasts, which are distributed throughout the body, including the lungs.
Prof. Erez: “Normally, fibroblasts play a critical role in wound healing and lung injury repair, but new research has showed that cancer successfully recruits them and induces them to create a favorable environment for it.” We sequenced all the genes expressed (transcriptome sequencing) in fibroblasts isolated from the lungs of mice used in a model of breast cancer metastasis as part of the current study.”
The researchers contrasted sequencing data from healthy lungs, lungs with micrometastases (very small metastases that cannot be detected by currently available clinical methods), and lungs with big metastases in an advanced stage of disease. According to the changes found from stage to stage, researchers were able to characterize for the first time the process occurring in the metastases’ microenvironment during the early phases of preparing the space for their reception.
Additionally, they identified the proteins that activate the’rewiring’ processes in fibroblasts and determined that one of the critical proteins in the process is MYC – a protein known for its role in promoting cancer cell division. This study discovered that MYC is also involved in the alterations that occur in fibroblasts as they prepare to receive metastases.
Prof. Erez summarizes: “We believe that in the future, our findings can aid in the identification of the metastatic process even before the disseminated cancer cells thrive and colonize the metastatic organ, with the purpose of providing prophylactic treatment. Such treatment, that will prevent the development of metastases, may save the lives of millions of people, worldwide.”
The study conducted was led by Prof. Neta Erez along with the research team of her laboratory, Dr. Ophir Shani and Dr. Yael Raz, as well as additional researchers from Tel Aviv University, Tel Aviv Medical Center (Ichilov), Sheba Medical Center and the Weizmann Institute.
Read more about: breast cancer