Pfizer testing use of pneumococcal vaccine alongside COVID-19 booster
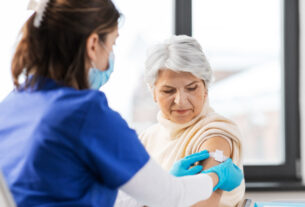
Pfizer administered the first set of jabs to volunteers of a new study of subjects 65 and older, exploring the co-administration of the company’s 20-valent pneumococcal conjugate vaccine (20vPnC) candidate when followed by a booster shot of Pfizer’s current COVID-19 vaccine, the company said on Monday.The 20vPnC vaccine candidate is currently in development to prevent invasive disease and pneumonia that can be caused by 20 serotypes of Streptococcus pneumonia in adults ages 18-years and older, Pfizer explained.Primarily, the study’s goal is to determine if it is safe to administer the 20vPnC alongside Pfizer-BioNTech COVID-19 vaccine, and the immune response after adding the pneumonia vaccine to the existing COVID-19 vaccine, Pfizer said. Volunteers will be receiving their third dose of the Pfizer vaccine over the course of the study.COVID-19 vaccines were previously recommended to be administered alone. But based on experience with non-COVID vaccines, the US Centers for Disease Control and Prevention has said COVID-19 shots and other vaccines can be given simultaneously or on the same day.The trial itself, includes 600 adults who were recruited from Pfizer’s phase 3 trial that preceded the worldwide rollout of the PFizer-BioNTech COVID-19 vaccine. Volunteers will have received their second dose of the vaccine at least six months prior to entering the co-administration study, the company said.Since then, the vaccine has been shipped out to over 91 countries, including Israel, who has inoculated over 5 million Israelis – the majority of its population – using the Pfizer vaccine.In December, the US Food and Drug Administration (FDA)accepted for a priority review Pfizer’s biologics license application for the investigational 20vPnC in adults over 18 and set an action date for a decision in June. The European Medicines Agency (EMA) accepted the company’s marketing authorization application for 20vPnC two months later.
if(window.location.pathname.indexOf(“656089”) != -1){console.log(“hedva connatix”);document.getElementsByClassName(“divConnatix”)[0].style.display =”none”;}Reuters contributed to this report.